Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
Advertisement
Scientific Reports volume 15, Article number: 499 (2025)
Metrics details
The objective of this study is to investigate the association between the Systemic Immune-Inflammation Index (SII) and cataracts. This cross-sectional study analyzed data from the 2005–2008 NHANES to examine the relationship between the SII and cataract prevalence. Covariates included age, race/ethnicity, gender, education level, marital status, Body Mass Index (BMI), smoking, alcohol consumption, hypertension, hyperlipidemia, and diabetes. Multivariable logistic regression was used to assess the association, while spline curve fitting explored potential non-linear relationships. Threshold analysis identified critical inflection points. To address age-related bias, Propensity Score Matching (PSM) was performed, aligning cataract patients with comparable non-cataract individuals for further evaluation. Our study included 3,623 participants, of whom 730 (20.15%) were diagnosed with cataracts. After adjusting for all covariates, multivariable logistic regression analysis demonstrated that elevated levels of the SII were significantly associated with increased odds of cataracts (Model1: OR = 1.56; 95%CI [1.33–1.85]; Model2: OR = 1.55; 95%CI [1.32–1.84]; Model3: OR = 1.57; 95%CI [1.33–1.86]). In the spline curve fitting model, the relationship between ln-SII and cataract prevalence was non-linear (P < 0.001), with a critical inflection point identified at an SII of 428.38. SII levels remained significantly associated with cataract prevalence following PSM adjustments (Model 1: OR = 1.48; 95% CI [1.21–1.80]; Model 2: OR = 1.48; 95% CI [1.21–1.80]; Model 3: OR = 1.46; 95% CI [1.20–1.78]). Elevated SII levels are associated with a higher prevalence of cataracts, underscoring the pivotal role of systemic inflammation in cataract development. These findings indicate that SII could serve as a valuable biomarker for assessing cataract risk, further emphasizing the significance of managing systemic inflammation as a potential strategy for cataract prevention.
The lens, positioned behind the iris yet in front of the vitreous body and retina, plays a crucial role in directing light onto the retina. Originating from ectodermal tissue, it comprises epithelial cells that produce lens fibers, causing the lens to thicken over time. Cataracts, characterized by reduced lens clarity, impair vision, decrease contrast sensitivity, alter color perception, and cause glare1,2,3. The World Health Organization reports that cataracts represent approximately 46% of the nearly 180 million global cases of visual impairments3. Although treatable, cataracts remain a leading cause of vision loss worldwide, posing significant public health challenges4,5. Factors such as aging, smoking, diabetes, and exposure to ultraviolet light contribute to the development of age-related cataracts6. Although cataract surgery can significantly improve vision, its affordability and the limited availability of surgeons in some regions continue to pose challenges7.
Immune cells play a crucial role in the systemic inflammatory response, which is implicated in a variety of diseases. Researchers have found that the combined count of lymphocytes, neutrophils, and platelets in peripheral blood provides a more accurate indication of inflammatory status8,9. Recent ophthalmologic studies have demonstrated a significant association between Systemic Immune-Inflammation Index (SII) and primary open-angle glaucoma10. Additionally, inflammation and immune responses are increasingly recognized as key factors in cataract development. Chronic inflammation induces oxidative stress, which promotes lens protein modification and ultimately leads to lens opacity11. Moreover, immune responses involving cytokines and inflammatory mediators have been implicated in the progression of cataracts.
Initially, SII was identified as a prognostic marker for gastrointestinal tumors in elderly patients12. Subsequent studies have also linked it to the occurrence of pseudophakic cystoid macular edema (PCME) following uncomplicated phacoemulsification cataract surgery in patients without risk factors, highlighting its potential as a predictive biomarker for PCME. This suggests its utility in enhancing clinical assessments and refining risk stratification13. However, the relationship between SII and cataracts remains unexplored. To address this gap, our research team conducted a population-based cross-sectional analysis using NHANES data to investigate the potential association between SII and cataract prevalence in adults.
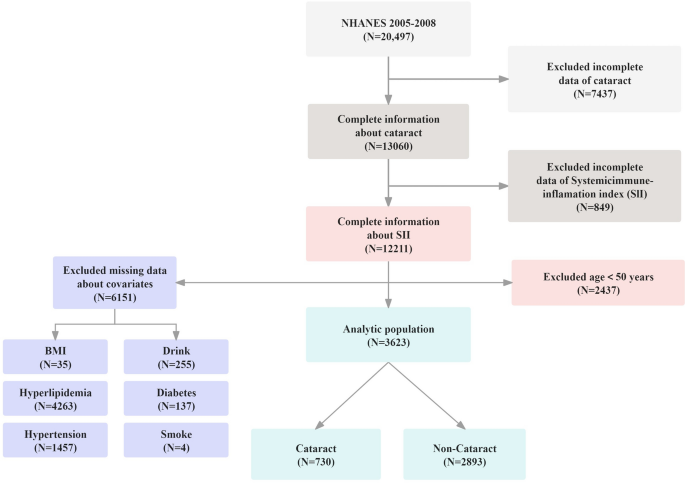
The NHANES is a cross-sectional study that collects comprehensive data on the health and nutritional status of U.S. households14. It employs a complex, stratified, multistage probability cluster sampling method to ensure an accurate representation of the U.S. population. For further insights into NHANES’s detailed methodology, please refer to their website at http://www.cdc.gov/nchs/nhanes/index.htm. Ethical clearance for the study was granted by the National Center for Health Statistics’ Ethics Committee, with all subjects providing written informed consent8. Our study excluded individuals with missing SII data, cataract information, or other necessary covariate details. Given the strong correlation between cataracts and age, we further excluded participants under the age of 50 to enhance the reliability and validity of our findings. This approach targeted an age group with a higher prevalence of cataracts, thereby minimizing the confounding effect of age on the association of the SII with cataract, and the effect of age on all potential confounders included in the model. Ultimately, the study population comprised 3,623 individuals, with the sample selection process illustrated in Fig. 1.
Screening process of the included studies.
According to the NHANES Vision Procedures Manual15, participants aged 20 and older were asked whether they had undergone cataract surgery. To minimize confounding factors associated with age, participants under the age of 50 were further excluded. Given the high accessibility and low barriers to cataract surgery in the United States, self-reported cataract surgery is considered a surrogate indicator for clinically significant cataracts. Participants completed a questionnaire (VIQ071: 2005–2008), which included the question, “Have you ever had cataract surgery?” An affirmative response was taken as indicative of cataracts16. This method of identifying cataracts is consistent with the methodologies used in previous studies16,17.
The Complete Blood Count (CBC) metrics employ the Beckman Coulter methodology, which integrates counting and sizing techniques, an automated system for diluting and mixing samples, and a single-beam photometer for measuring hemoglobin levels. White Blood Cell (WBC) differentials utilize VCS technology. Positioned within the NHANES mobile examination center (MEC), the Beckman Coulter DxH 800 instrument conducts CBC analyses on participant blood samples, providing a comprehensive cell distribution profile. The SII is derived from these three circulating immune cell types and is calculated using the formula: platelets (PC) × neutrophils (NC) / lymphocytes (LC) 18.
Demographic variables such as age, race/ethnicity, gender, education level, marital status, and Body Mass Index (BMI) were selected as covariates in our study. This demographic information was collected via computer-assisted personal interviews19. The comorbidities considered included hypertension, hyperlipidemia, and diabetes mellitus. Hypertension was defined as either a doctor’s diagnosis, the use of antihypertensive medication, or having a systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg. Hyperlipidemia was identified in participants with a doctor’s diagnosis, those taking lipid-lowering medications, or those with a total cholesterol level ≥ 240 mg/dL during NHANES assessments. Diabetes mellitus was determined by a doctor’s diagnosis, the use of glucose-lowering medication or insulin, or an HbA1c level ≥ 6.5% during NHANES testing. In terms of lifestyle, the covariates included were smoking and alcohol consumption. Smoking was categorized into two groups: non-smokers (those who have never smoked or have smoked fewer than 100 cigarettes in their lifetime) and smokers (those who have smoked at least 100 cigarettes in their lifetime)20. Similarly, alcohol consumption was divided into two categories: non-drinkers (those who have never consumed alcohol or have consumed fewer than 12 drinks in their lifetime) and drinkers (those who have consumed at least 12 drinks in their lifetime)21.
Data analysis was performed using R2 and EmpowerStats software, developed by X&Y Solutions, Inc., based in Boston, MA, and available at http://www.empowerstats.com. This analysis accounted for the complex sampling framework of NHANES by incorporating sampling weights, strata, and primary sampling units. Continuous variables were presented as means ± standard errors (SE), and categorical variables as percentages ± SE. Chi-square tests or T-tests were utilized to assess demographic differences.
The SII data exhibited a right-skewed distribution, necessitating a natural logarithm transformation for statistical analysis. Logistic regression models were utilized to assess the association between SII levels and cataract risk. Model 1 adjusted for age, gender, race, and BMI. Model 2 included additional adjustments for education level, marital status, smoking, and alcohol consumption. Model 3, building on Model 1, also accounted for diabetes, hypertension, and hyperlipidemia. Quantile regression analyses were conducted to further explore these associations. The logistic regression results were visually represented through forest plots, and smooth curve fitting was utilized to investigate potential relationships between SII levels and cataract risk. Analysis of threshold and saturation effects were performed to identify the optimal inflection point. In order to mitigate potential age-related effects on the outcomes, cataract patients and non-cataract individuals were matched in a 1:1 ratio based on average age in the propensity score matching (PSM) analysis. Statistical significance was established at p-values less than 0.05.
The study encompassed a total of 3,623 participants, including 2,893 individuals without cataracts and 730 who were diagnosed with cataracts following screening. The characteristics of these participants are summarized in Table 1.
The average age of participants was 65.9 years, consisting of 1,786 males (49.30%) and 1,837 females (50.70%). Participants who had undergone cataract surgery were predominantly older, unmarried females with lower levels of education. Additionally, it was observed that patients with a history of smoking or alcohol consumption were more susceptible to developing cataracts. Furthermore, participants diagnosed with hypertension, hyperlipidemia, or diabetes exhibited a higher incidence of cataract formation. Table 1 demonstrates that individuals with cataracts presented higher SII scores, corroborating our initial hypothesis.
We performed a weighted multivariate logistic regression analysis (illustrated in Fig. 2), which revealed a significant positive association between elevated ln-SII scores and the likelihood of cataract prevalence. This association was consistently significant across all models: Model 1 (OR = 1.56; 95% CI = 1.33–1.85, p < 0.001), Model 2 (OR = 1.55; 95% CI = 1.32–1.84, p < 0.001), and Model 3 (OR = 1.57; 95% CI = 1.33–1.86, p < 0.001). Further analysis using smoothing spline techniques delineated a non-linear relationship between ln-SII and the odds of having cataracts across various covariates (as depicted in Fig. 3, p < 0.001). Additionally, threshold and saturation effect analyses identified an inflection point at an ln-SII of 6.06 (equivalent to an SII of 428.38). Below this threshold, the association between SII and the prevalence of cataracts is weaker; however, when SII exceeds this value, its positive correlation with the prevalence of cataracts significantly strengthens.
Forest plot of logistic regression results. Note: Model 1: adjusted for Gender; Age; Race; BMI. Model 2: adjusted for Gender; Age; Race; BMI; Education; Marital Status; Smoke; Drink. Model 3: adjusted for Gender; Age; Race; BMI; Hypertension; Hyperlipemia; Diabetes.
Relationship between ln-SII and cataract prevalence. Note: The red solid line represents a smoothed curve fit of SII to cataract prevalence. The blue dashed line represents the 95% confidence interval of the smoothed curve fit.
For sensitivity analysis, we transformed ln-SII from a continuous variable into categorical quartiles, as depicted in Table 2. Compared to the lowest quartile (Q1), participants in the highest quartile (Q4) exhibited 96% higher odds of having cataracts (OR = 1.96; 95% CI = 1.51–2.54, p < 0.001) in Model 1, 95% higher odds in Model 2 (OR = 1.95; 95% CI = 1.49–2.53, p < 0.001), and 99% higher odds in Model 3 (OR = 1.99; 95% CI = 1.53–2.59, p < 0.001). Further analysis using smoothing spline techniques demonstrated a linear relationship between ln-SII (values above 6.06) and the odds of developing cataracts, adjusted for all covariates, as illustrated in Fig. 4 (p < 0.001). This analysis substantiates the strong association between elevated ln-SII (values above 6.06) levels and increased odds of having cataracts..
Relationship between ln-SII(values above 6.06) and cataract prevalence. Note: Based on the turning point (ln-SII > 6.06) in Fig. 3, the linear relationship between ln-SII and cataract adjusted for all covariates was further explored.
Given the age and numerical discrepancies between the cataract and normal groups, a 1:1 propensity score matching (PSM) analysis was conducted based on age to mitigate age-related effects. A total of 1,254 participants were enrolled and classified into the cataract and non-cataract groups. The baseline characteristics of each group, post-PSM, are presented in Table 3. Significant differences in the SII were observed between the groups after PSM, with the cataract group displaying elevated SII levels (P < 0.001). Following this, the logistic regression model, which assessed the relationship between SII and the incidence of cataracts post-PSM, indicated a significant positive correlation between higher SII scores and the prevalence of cataracts. This correlation remained consistently significant across all models (Model1:1.48 (1.21, 1.80); Model2:1.48 (1.21, 1.80); Model3:1.46 (1.20, 1.78), adhering to the inclusion criteria and details that align with those previously described, as further detailed in Supplementary Table 1.
In this study, we leveraged data from the NHANES database to investigate the potential link between the SII and cataract development. Our findings from this nationally representative cross-sectional analysis revealed a nonlinear relationship between SII levels and cataract prevalence. We identified a critical inflection point for ln-SII at 6.06 (SII = 428.38), beyond which SII levels positively correlate with increased cataract prevalence. To the best of our knowledge, this is the inaugural study to explore the association between SII and cataracts, offering a novel perspective in the understanding of this relationship.
Systemic inflammation can be measured through various biochemical or hematological markers commonly assessed in routine blood analyses, or via ratios derived from these markers22. The SII, a novel and consistent marker of inflammation, is computed using the formula: PC × NC / LC8.
Elevated SII levels indicate an inflammatory environment marked by increased NC and decreased LC, potentially contributing to the onset and progression of various diseases. Such elevation not only reflects an inflammatory state but may also indicate a disruption in immune regulation. Cells like NC and LC play crucial roles in managing inflammation and immune responses. Consequently, a high SII may denote persistent immune activation, a condition frequently observed in the pathogenesis of various chronic diseases, including age-related disorders such as cataracts23.
The connection between inflammatory states and cataract formation may be mediated by inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6)24. Released during inflammatory responses, these cytokines directly alter the intraocular environment, promoting oxidative damage and apoptosis in lens epithelial cells, thereby accelerating the development of cataracts. Concurrently, elevated levels of SII lead to an increase in inflammatory mediators within the bloodstream. These mediators, penetrating the eye via ocular circulation, trigger inflammatory pathways within the lens. This activation induces further cellular responses, including the release of additional cytokines, which exacerbate oxidative stress and cellular damage in the lens25.
Alterations in the SII may also indicate the infiltration status of immune cells, particularly NC and LC, within the eye. The activation and subsequent infiltration of these cells can directly damage the lens or indirectly lead to lens cell injury and death through the release of inflammatory mediators and enzymes. Such processes significantly accelerate the development of cataracts.
Oxidative stress is intricately linked to mitochondrial function. Under conditions of inflammation and elevated SII, oxidative stress can escalate, leading to mitochondrial dysfunction—a key factor in the development of cataracts. This dysfunction may decrease ATP production and disrupt cellular metabolism, impairing the normal functioning and viability of lens cells. Furthermore, mitochondrial dysfunction can enhance the production of reactive oxygen species (ROS) within cells, further exacerbating oxidative stress26.
Additionally, prolonged inflammatory responses and oxidative stress can deplete the body’s antioxidant defense mechanisms. Notably, the activities of antioxidant enzymes, such as superoxide dismutase (SOD) and glutathione peroxidase (GP), may diminish. Concurrently, the overall levels of antioxidants may decrease, impairing the eye’s capacity to neutralize ROS. This reduction in antioxidant defense can lead to increased oxidative damage to the lens, exacerbating conditions conducive to cataract formation.
Considering the established correlation between the SII and cataracts, future research should focus on therapeutic strategies that target inflammation and oxidative stress. Potential approaches could include the development of novel pharmacological agents or nutritional supplements designed to mitigate inflammatory responses, enhance antioxidant defenses, or directly scavenge ROS. Additionally, subsequent studies ought to evaluate the utility of SII in clinically assessing cataract risk and monitoring disease progression. With an enhanced understanding of the precise relationship between SII and cataract formation, SII could serve as a pivotal marker for predicting cataract risk and directing appropriate intervention strategies.
Our study is bolstered by a substantial, nationally representative sample and adjusts for critical demographic, examination, and laboratory factors, thereby enhancing the credibility and generalizability of our findings. However, the study also exhibits limitations, chiefly its cross-sectional design, which constrains our capacity to establish causality. Furthermore, the NHANES survey administrators did not thoroughly account for the age-specific characteristics of cataracts, including comprehensive records of bilateral conditions during ophthalmological screenings. To address these challenges, future research employing a robust sample size is essential to clarify causal relationships. Additionally, it is imperative to further investigate the possible association between SII and the history of bilateral cataract surgery.
This study, drawing on data from the NHANES database, unveils new insights into the correlation between the SII and cataract development. Typically, SII is positively correlated with inflammatory conditions, such as cataract. A critical SII value of 428.38 emerges with significant clinical implications. These findings propose SII as a novel biomarker for the risk assessment and early prevention of cataracts, highlighting the pivotal role of systemic inflammation in cataract pathogenesis.
Publicly available datasets were analyzed in this study. This data can be found at: https://www.cdc.gov/nchs/nhanes/index.htm.
Systemic immune-inflammation index
National health and nutrition examination survey
Propensity score matching
Complete blood count
White blood cell
Mobile examination center
Body mass index
Standard errors
Odds ratio
Tumor necrosis factor-alpha
Interleukin-6
Reactive oxygen species
Super oxide dismutase
Glutathione peroxidase
Lymphocytes
Neutrophils
Platelets
Pseudophakic cystoid macular edema
Logarithmic
Asbell, P. A. et al. Age-related cataract. Lancet 365, 599–609 (2005).
Article PubMed Google Scholar
Cedrone, C. et al. Prevalence and incidence of age-related cataract in a population sample from Priverno, Italy. Ophthalmic Epidemiol. 6, 95–103 (1999).
Article CAS PubMed Google Scholar
Demmin, D. L. & Silverstein, S. M. Visual impairment and mental health: unmet needs and treatment options. Clin. Ophthalmol. 14, 4229–4251 (2020).
Article PubMed PubMed Central Google Scholar
Cedrone, C. et al. Prevalence of blindness and low vision in an Italian population: A comparison with other European studies. Eye 20, 661–667 (2006).
Article CAS PubMed Google Scholar
Hashemi, H. et al. Ocular components during the ages of ocular development. Acta Ophthalmol. 93, e74–e81 (2015).
Article PubMed Google Scholar
Guo, J. et al. Aging and aging-related diseases: From molecular mechanisms to interventions and treatments. Sig. Transduct Target Ther. 7, 1–40 (2022).
Article Google Scholar
Mailu, E. W., Virendrakumar, B., Bechange, S., Jolley, E. & Schmidt, E. Factors associated with the uptake of cataract surgery and interventions to improve uptake in low- and middle-income countries: A systematic review. PLoS ONE 15, e0235699 (2020).
Article CAS PubMed PubMed Central Google Scholar
Mahemuti, N. et al. Association between Systemic Immunity-Inflammation Index and Hyperlipidemia: A Population-Based Study from the NHANES (2015–2020). Nutrients 15, 1177 (2023).
Article CAS PubMed PubMed Central Google Scholar
Zhao, Y. et al. Association between systemic immune-inflammation index and metabolic syndrome and its components: results from the National Health and Nutrition Examination Survey 2011–2016. J. Transl. Med. 21, 691 (2023).
Article CAS PubMed PubMed Central Google Scholar
Associations between blood cell profiles and primary open-angle glaucoma: A retrospective case-control study – PubMed. https://pubmed.ncbi.nlm.nih.gov/32018245/.
Lim, J. C., Caballero Arredondo, M., Braakhuis, A. J. & Donaldson, P. J. Vitamin C and the Lens: New insights into delaying the onset of cataract. Nutrients 12, 3142 (2020).
Article CAS PubMed PubMed Central Google Scholar
He, L., Xie, X., Xue, J., Xie, H. & Zhang, Y. Association of the systemic immune-inflammation index with all-cause mortality in patients with arteriosclerotic cardiovascular disease. Front. Cardiovasc. Med. https://doi.org/10.3389/fcvm.2022.952953 (2022).
Article PubMed PubMed Central Google Scholar
Kocamış, S. İ, Boz, A. A. E. & Özdemir, İ. Systemic immune-inflammation index could be associated with pseudophakic cystoid macular edema after an uneventful phacoemulsification surgery in patients without risk factors. BMC Ophthalmol. 22, 378 (2022).
Article PubMed PubMed Central Google Scholar
NHANES Questionnaires, Datasets, and Related Documentation. https://wwwn.cdc.gov/nchs/nhanes/ContinuousNhanes/Default.aspx?BeginYear=2005.
VIQ_D. https://wwwn.cdc.gov/Nchs/Nhanes/2005-2006/VIQ_D.htm.
Kisic, B., Miric, D., Zoric, L., Ilic, A. & Dragojevic, I. Antioxidant capacity of lenses with age-related cataract. Oxid. Med. Cell Longev. 2012, 467130 (2012).
Article PubMed PubMed Central Google Scholar
Park, S. J., Lee, J. H., Kang, S. W., Hyon, J. Y. & Park, K. H. Cataract and cataract surgery: Nationwide prevalence and clinical determinants. J. Korean Med. Sci. 31, 963–971 (2016).
Article PubMed PubMed Central Google Scholar
Hu, B. et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin. Cancer Res. 20, 6212–6222 (2014).
Article ADS CAS PubMed Google Scholar
Ahluwalia, N. Nutrition monitoring of children aged birth to 24 Mo (B-24): Data collection and findings from the NHANES. Adv. Nutr. 11, 113–127 (2020).
Article PubMed Google Scholar
NHANES 2007–2008: Smoking – Cigarette Use Data Documentation, Codebook, and Frequencies. https://wwwn.cdc.gov/Nchs/Nhanes/2007-2008/SMQ_E.htm.
ALQ_E. https://wwwn.cdc.gov/Nchs/Nhanes/2007-2008/ALQ_E.htm.
Sylman, J. L. et al. The predictive value of inflammation-related peripheral blood measurements in cancer staging and prognosis. Front. Oncol. 8, 78 (2018).
Article PubMed PubMed Central Google Scholar
Ferrucci, L. & Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 15, 505–522 (2018).
Article CAS PubMed PubMed Central Google Scholar
Liu, S. et al. Protection of human lens epithelial cells from oxidative stress damage and cell apoptosis by KGF-2 through the Akt/Nrf2/HO-1 pathway. Oxid. Med. Cell Longev. 2022, 6933812 (2022).
PubMed PubMed Central Google Scholar
Tjalkens, R. B., Popichak, K. A. & Kirkley, K. A. Inflammatory activation of microglia and astrocytes in manganese neurotoxicity. Adv. Neurobiol. 18, 159–181 (2017).
Article PubMed PubMed Central Google Scholar
Nunnari, J. & Suomalainen, A. Mitochondria: In sickness and in health. Cell 148, 1145–1159 (2012).
Article CAS PubMed PubMed Central Google Scholar
Download references
The authors would like to thank all reviewers for their valuable comments.
The study described was supported by grants from a Key Project and a Lab Project at Chongqing Three Gorges Medical College, China (SYS20210021), and a project supported by the Chongqing Education Commission Science and Technology Research Program (KJQN202302715).
Xiang Li, Guo-lei Du and Shi-Nan Wu, contributed equally to this work.
Eye Institute & Affiliated Xiamen Eye Center, School of Medicine, Xiamen University, XiaMen, China
Xiang Li & Shi-Nan Wu
Chongqing Key Laboratory of Development and Utilization of Genuine Medicinal Materials in Three Gorges Reservoir Area, Chongqing Three Gorges Medical College, No. 366, Tian Xing Rd, Bai’anba, Wanzhou, chongqing, China
Xiang Li & Jia-feng Tang
Weihai Institute for Bionics-Jilin University, Weihai, China
Guo-lei Du & Si-Qi Zhang
The First Affiliated Hospital of Xi’an Jiao Tong University, Shaanxi, 710061, China
Yi-qing Sun
Kunming Medical University, Kunming, 650500, Yunnan, China
Zhi-Jie Zhang
You can also search for this author in PubMed Google Scholar
You can also search for this author in PubMed Google Scholar
You can also search for this author in PubMed Google Scholar
You can also search for this author in PubMed Google Scholar
You can also search for this author in PubMed Google Scholar
You can also search for this author in PubMed Google Scholar
You can also search for this author in PubMed Google Scholar
Xiang Li conceived the research idea. Xiang Li, Guo-lei Du, Shi-Nan Wu, Yi-qing Sun, Si-Qi Zhang, Zhi-Jie Zhang conducted data cleaning and literature review. Xiang Li, Guo-lei Du, Shi-Nan Wu, and Si-Qi Zhang, contributed to drafting and critically revising the work for intellectual content. Xiang Li, Guo-lei Du, Shi-Nan Wu, and Si-Qi Zhang, conducted the analysis and created the figures and tables. Jia-feng Tang provided a critical review of the manuscript. All authors have read and approved the manuscript.
Correspondence to Jia-feng Tang.
The authors declare no competing interests.
Considering that the NHANES database is publicly accessible, and patient records are anonymous and de-identified, it does not involve informed consent or ethical approval.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
Reprints and permissions
Li, X., Du, Gl., Wu, SN. et al. Association between systemic immune inflammation index and cataract incidence from 2005 to 2008. Sci Rep 15, 499 (2025). https://doi.org/10.1038/s41598-024-84204-7
Download citation
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-84204-7
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Advertisement
© 2025 Springer Nature Limited
Sign up for the Nature Briefing: Translational Research newsletter — top stories in biotechnology, drug discovery and pharma.
