Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
Advertisement
Scientific Reports volume 14, Article number: 31270 (2024)
Metrics details
Eczema is a common chronic skin condition. Previous studies indicated the dietary factors, such as calcium intake, might influence the onset and progression of eczema in the population of gravidas and infants. However, there was no studies on the correlation between dietary calcium and the adult population. In this study, we aim to investigate the correlation between dietary calcium intake and the prevalence of eczema in adults. The characteristics of adults (≥ 18 years) were collected from the National Health and Nutrition Examination Survey (NHANES) 2005–2006 database. Dietary calcium intake was assessed using the 24-hour dietary recall method. The prevalence of eczema was determined through an allergy questionnaire. Logistic regression modeling was applied to analyze the correlation between dietary calcium intake and eczema prevalence. Restricted cubic spline (RCS) was used to investigate the nonlinear relationship between calcium intake and eczema. A two-stage linear regression model was used to calculate the critical effect of calcium intake on the prevalence of eczema by smoothed curve fitting. Subgroup analyses were performed to explore the effect of different demographic characteristics on the relationship between dietary calcium intake and eczema. Results In this cross-sectional study, we collected 4086 adult samples. There were 1930 males (46.9%) and 2156 females (53.1%), at the average age of 46.7 years, and 266 participants (7.6%) were diagnosed with eczema. Logistic regression results showed there was a significant difference between the third quartile group and eczema compared to the 1st quartile group of dietary calcium (OR: 1.913, 95% CI: 1.024–3.576, P = 0.043). The RCS showed an inverted U-shaped correlation between dietary calcium intake and eczema prevalence (non-linear P-value < 0.05). An increase in calcium intake was associated with an increase in eczema prevalence when the logarithmic value of dietary calcium intake was below 7.089 (OR: 1.790, 95% CI: 1.006–3.183, P = 0.048). These data indicated there was an inverted U-shaped correlation between dietary calcium intake and the prevalence of eczema, which suggested moderate reduction of calcium intake might be beneficial in the incidence of eczema. Further prospective studies are needed to explore causal relationships and optimal calcium intake levels to prevent eczema.
Eczema is a common chronic, recurrent skin disease primarily characterized by severe itching and dry skin. According to the statistics, approximately 20% of children and 10% of adults worldwide suffer from eczema, with the incidence rate showing an increasing trend1,2. This condition significantly impacts patients’ quality of life and mental health. The etiology and pathogenesis of eczema are complex, involving multiple factors such as genetics, immunity, environment, and psychology3. Current research suggests that eczema is associated with impaired skin barrier function, abnormal immune responses, microbial infections, and environmental factors (e.g., allergens and pollutants)4,5. However, the exact cause of eczema remains unclear, necessitating further research to elucidate its pathological mechanisms.
Recent studies indicated that dietary factors not only affect overall health but might also influence skin health through various pathways. For instance, certain nutrients in the diet, such as folic acid, vitamin D, omega-3 fatty acids, and antioxidants, were believed to be associated with the occurrence and development of eczema6,7,8,9,10. Calcium is an essential mineral for the human body, involved in various physiological functions, including the formation of bones and teeth, nerve conduction, muscle contraction, and blood clotting. Beyond these known functions, calcium also plays a crucial role in maintaining skin barrier function and the differentiation of keratinocytes11. Additionally, calcium is a significant component of the extracellular matrix, and its deficiency can affect epidermal cell differentiation and renewal, leading to a thinner stratum corneum and rough skin12. Studies have shown that calcium deficiency may impair skin barrier function, resulting in symptoms such as rashes, eczema, urticaria, and folliculitis13,14.
Recent research indicated that reduced intake of dietary calcium or consumption of calcium-rich foods (such as legumes and dairy products) was associated with an increased prevalence of eczema10,14,15. However, some studies suggested that decreased expression of calcium or calcium-related proteins might enhance skin anti-allergic properties, reducing the incidence of eczema16,17,18,19. Given that previous research primarily focused on pregnant women or children and produced inconsistent findings on the association between calcium intake and eczema, the relationship between dietary calcium intake and eczema in adults remained inadequately explored. Therefore, this study aimed to further investigate the association between dietary calcium intake and eczema using data from NHANES 2005–2006.
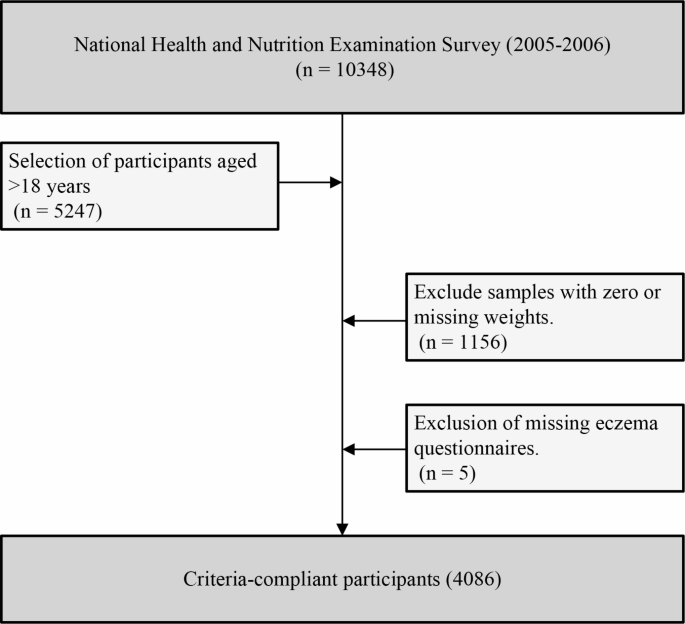
The data for this study were derived from the National Health and Nutrition Examination Survey (NHANES) 2005–2006, a nationally representative health and nutrition survey conducted by the Centers for Disease Control and Prevention (CDC)20. This study employed a cross-sectional design, adhering strictly to the guidelines of the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement. The study included adult individuals (≥ 18 years) who participated in the NHANES survey during 2005–2006, excluding those with missing sample weights and questionnaires (Fig. 1). All participants provided written informed consent, and the data collection and usage were approved by the Research Ethics Review Board of the National Center for Health Statistics.
Flow chart of the study design.
Dietary data in this study were collected using the 24-hour dietary recall method. Trained interviewers conducted face-to-face interviews with participants using standardized interview procedures to record all food and beverage intake in the past 24 h. The interview process employed the multiple-pass method, which included quick recall, detailed inquiry, and review and confirmation to ensure data comprehensiveness and accuracy. The dietary analysis system of the United States Department of Agriculture (USDA) was used to convert the reported food and beverage intake into nutrient data, specifically calculating the daily calcium intake (in mg), which included calcium from all foods, beverages, and supplements21,22. Participants underwent a second 24-hour dietary recall interview by phone 3 to 10 days later. The average calcium intake from the two 24-hour dietary recalls was used in this study. Additionally, this study collected data on the intake of other key nutrients, including carbohydrates, protein, and total energy.
The prevalence of eczema among participants was collected using an allergy questionnaire. Participants were asked the following question, “Has a doctor or other healthcare professional ever told you that you had eczema? (Yes/No)”. If the answer was yes, they were categorized as having eczema. This definition is consistent with previous NHANES survey results9,17,23.
To adjust for potential confounding factors in the multivariable models, this study collected basic demographic information including gender, age, race, marital status, education level, height, and weight. Laboratory tests included measurements of vitamin D3, serum IgE, C-reactive protein (CRP), white blood cells, neutrophils, eosinophils, monocytes, and red blood cells. Health status variables included physical activity, smoking, alcohol consumption, asthma, and diabetes. Body mass index (BMI) was calculated as weight (kg) divided by the square of height (m). Physical activity was assessed based on metabolic equivalent (MET) values and categorized as poor (< 600 MET minutes/week), ideal (≥ 8000 MET minutes/week), and moderate (600–7999 MET minutes/week)24. Smoking status was defined by the quantity and duration of lifetime smoking and categorized as never, former, and current smokers25. Alcohol consumption was defined based on the amount of daily drinking throughout the year and categorized as never, former, mild, moderate, or heavy drinker26. Asthma status was determined if participants answered affirmatively to having been diagnosed with asthma by a doctor or currently having asthma27. Diabetes status was defined based on self-report, current use of antidiabetic medications, or fasting blood glucose and glycated hemoglobin levels exceeding normal values26.
Statistical analyses were conducted using R software (version 4.3.1), with all analyses weighted using the “survey” package to account for the complex sampling design of NHANES28,29. Initially, the sample was divided into quartiles based on dietary calcium intake, and baseline characteristics were compared across these groups. Categorical variables were expressed as frequencies and weighted percentages, and group comparisons were made using the Rao-Scott chi-square test. Continuous variables were expressed as weighted means and standard deviations, and group comparisons were performed using one-way analysis of variance (ANOVA). To further investigate whether there is a significant difference between dietary calcium intake in the study population and the optimal intake level recommended by the National Institutes of Health (NIH), a one-sample T-test was conducted. Subsequently, logistic regression models were applied to analyze the association between dietary calcium intake and the prevalence of eczema, adjusting for potential confounders. A 10-fold cross-validation was also used to assess whether the models were overfitted. Restricted cubic splines (RCS) were used to investigate any potential nonlinear relationship between calcium intake and eczema. If a nonlinear relationship was identified, piecewise linear regression models were employed to calculate the threshold effect of calcium intake on eczema prevalence through smooth curve fitting. Finally, subgroup analyses were conducted to explore the influence of different demographic characteristics (such as gender and race) on the relationship between dietary calcium intake and eczema. In all analyses, a two-sided P value of < 0.05 was considered statistically significant.
This study included 4,086 eligible adult participants, comprising 1,930 males (46.9%) and 2,156 females (53.1%), with an average age of 46.7 years. Among them, 266 participants (7.6%) were diagnosed with eczema. Participants were divided into four groups based on the quartiles of dietary calcium intake: Q1 (< 496 mg), Q2 (496 mg to 770 mg), Q3 (770 mg to 1151.5 mg), and Q4 (> 1151.5 mg) (Table 1). Analysis revealed significant differences (P < 0.05) among the four groups in terms of age, gender, race, education level, vitamin D3, neutrophils, red blood cells, energy, protein, carbohydrate, smoking status, and drinking status. However, no significant differences (P > 0.05) were observed for BMI, marital status, IgE, C-reactive protein, leukocytes, lymphocytes, monocytes, eosinophils, basophils, physical activity, diabetes mellitus, asthma, and eczema. Notably, despite the non-significant P values, a higher distribution of participants with eczema was observed in the Q3 group, with 74 cases (9.4%), compared to the Q1 group, which had the lowest distribution of participants with eczema, with 66 cases (6.1%).
The one-sample T-test indicated a statistically significant difference between dietary calcium intake in various gender and age groups within this study population and the optimal intake levels recommended by the NIH. Among women of all age groups, actual dietary calcium intake was lower than the recommended intake level (P < 0.05). For men aged 25 ~ 65, actual dietary calcium intake was higher than the recommended intake level, while elderly men had a lower intake than recommended (P < 0.05). Additionally, postmenopausal women (50 ~ 65 years) had the highest prevalence of eczema (Table 2).
First, logistic regression analysis examined the association between dietary calcium intake and eczema (Table 3). In the unadjusted model, no significant association was observed between dietary calcium intake and eczema, whether calcium intake was considered a categorical or continuous variable. In Model 1, adjustments were made for age, sex, ethnicity, marital status, drinking status, smoking status, diabetes mellitus, asthma, energy intake, protein intake, and carbohydrate intake. The adjusted model indicated that compared to the Q1 group, the Q3 group was associated with an increased prevalence of eczema (OR: 1.890, 95% CI: 1.033–3.458, P = 0.040). Further adjustment for vitamin D3, Ig-E, C-reactive protein, neutrophils, red blood cells, and eosinophils in Model 2 showed a significant association between the Q3 group and eczema (OR: 1.913, 95% CI: 1.024–3.576, P = 0.043). Notably, dietary calcium intake as a continuous variable did not significantly affect eczema in any of the three models. Cross-validation showed that none of the models were overfitted (sTable 1). Additionally, no significant trend P-value was observed, suggesting a potential nonlinear relationship between dietary calcium intake and eczema. The Restricted Cubic Spline (RCS) curve indicated a U-shaped association between dietary calcium intake and eczema (non-linear P-value < 0.05) (Fig. 2). Using a two-piece linear regression model, a threshold effect was calculated, with the log of dietary calcium intake indicating a breakpoint at 7.089. When the log of dietary calcium intake was less than 7.089, an association between dietary calcium intake and eczema was observed (OR: 1.790, 95% CI: 1.006–3.183, P = 0.048).
Dose-response relationship of dietary calcium intake about eczema. Adjusted for age, sex, ethnicity, marital status, drinking status, smoking status, energy intake, protein intake, diabetes mellitus, asthma, carbohydrate intake, vitamin D3, Ig-E, C-reactive protein, neutrophils, red blood cells, and eosinophils.
The subgroup analysis further explored the relationship between dietary calcium intake and eczema risk in different populations (Fig. 3). These analyses indicated a significant association between increased dietary calcium intake and eczema in specific populations, including postmenopausal women (OR: 4.382, 95% CI: 1.596–12.031, P = 0.007), elderly women (OR: 3.934, 95% CI: 1.068–14.489, P = 0.041), Non-Hispanic Whites (OR: 2.449, 95% CI: 1.270–4.724, P = 0.011), individuals without diabetes (OR: 2.142, 95% CI: 1.253–3.663, P = 0.008), individuals with asthma (OR: 2.546, 95% CI: 1.035–6.259, P = 0.043), non-smokers (OR: 2.002, 95% CI: 1.153–3.476, P = 0.017), former drinkers (OR: 4.429, 95% CI: 1.219–16.094, P = 0.027), and light drinkers (OR: 2.964, 95% CI: 1.125–7.805, P = 0.030). Notably, among pregnant women (OR: 0.176, 95% CI: 0.042–0.731, P = 0.030), increased dietary calcium intake was associated with a reduced risk of eczema.
Subgroup analysis of the relationship between dietary calcium intake and eczema in the American adult population. Note: log value for dietary calcium intake < 7.089. Adjusted for age, marital status, energy intake, protein intake, carbohydrate intake, vitamin D3, Ig-E, C-reactive protein, neutrophils, red blood cells, and eosinophils.
This study indicates that dietary calcium intake is below the recommended level among American adults. However, calcium intake at the recommended daily intake level may be associated with an increased prevalence of eczema in specific populations. Additionally, there is an inverted U-shaped relationship between dietary calcium intake and eczema prevalence. When the logarithmic value of dietary calcium intake is below 7.089, increased calcium intake is associated with a higher prevalence of eczema in populations such as postmenopausal women and elderly women. However, in pregnant women, increased dietary calcium intake is associated with a reduced risk of eczema.
The potential relationship between dietary calcium intake and eczema has been preliminarily explored in some studies. Previous research has mainly focused on specific populations, such as children and pregnant women, finding that calcium intake levels may have an impact on skin health and the immune system. However, due to the limitations of previous study populations, our findings differ from several earlier studies. A study on a Manitoba child cohort indicated that children with eczema had significantly lower calcium intake and consumption during adolescence14. Another study on children in Hong Kong found that low dietary calcium intake was associated with increased eczema prevalence7. In a cohort study on Japanese pregnant women, higher calcium intake during pregnancy was not significantly associated with the risk of eczema10. A case-control study in Spain found that asthmatic adults had higher calcium intake than the general population, but the difference was not statistically significant30. These studies suggest that calcium intake has a certain impact on skin health and immune regulation, but the consistency of these results across different populations is limited. Moreover, in our study, increased calcium intake was associated with a reduced risk of eczema among pregnant women. However, a study on Japanese pregnant women found that moderate calcium intake was associated with the prevalence of asthma18. Previous research has also indicated that pregnant women’s immune systems undergo changes to adapt to fetal growth and development31. Under these conditions, moderate calcium intake may have a protective role in immune regulation, skin health maintenance, and eczema prevention. Additionally, increased calcium intake may enhance skin barrier integrity and anti-inflammatory capacity in pregnant women, thus reducing the incidence of allergic skin conditions.
Notably, some studies on calcium-related nutrient intake and allergic diseases indirectly support similar findings. A prospective cohort study in Finland indicated that vitamin D supplementation during infancy was associated with an increased risk of allergic diseases (asthma or allergic rhinitis) in adulthood32. A study in the UK on women in late pregnancy found that 25(OH)-vitamin D concentrations above 75 nmol/L were associated with an increased risk of allergic diseases in infants33A study examining the relationship between serum vitamin D levels and eczema prevalence in US adults found an inverted U-shaped relationship between the two, similar to our findings17Vitamin D and calcium have closely related metabolism and functions, with vitamin D promoting calcium absorption to support bone health and various physiological functions34.
In the pathogenesis of eczema, Th2 (T-helper 2) cells play a dominant role35. Th2 cells produce cytokines such as IL-4, IL-5, and IL-13, which promote B cells to produce IgE, leading to allergen sensitization. These cytokines also recruit and activate eosinophils, contributing to allergic inflammation, and impair the skin barrier by reducing the production of proteins and lipids necessary for barrier function36,37. In chronic eczema, Th1 and Th17 cells are also involved. Th1 cells produce IFN-γ, which activates macrophages and sustains inflammation, while Th17 cells produce IL-17, recruiting neutrophils and further contributing to chronic inflammation and barrier dysfunction38,39. The pathogenesis of eczema is also related to a complex network of cytokines. IL-31 produced by Th2 cells directly induces itch, while IL-22 produced by Th17 cells disrupts skin barrier function by affecting keratinocyte differentiation and proliferation40,41. Our findings from the NHANES-based study suggest for the first time that increased dietary calcium intake may elevate the risk of eczema. The potential mechanisms could align with recent reports from animal models. Elevated calcium ion levels can affect immune cell function and potentially exacerbate the dysregulation observed in eczema. Calcium ions act as secondary messengers in immune cells, regulating various functions such as T cell activation42. Calcium influx is crucial for T cell receptor (TCR) signaling, leading to T cell activation and proliferation. Calcium also enhances TCR signaling, which may increase Th2 and Th17 responses43,44. Additionally, calcium signaling influences cytokine production. Elevated calcium levels can enhance the production of IL-4, IL-5, and IL-13 by Th2 cells, and IL-17 by Th17 cells, thus exacerbating inflammation45. Elevated calcium levels can also disrupt skin barrier function by impairing keratinocyte differentiation. Excessive calcium can alter the differentiation process of keratinocytes, leading to skin barrier dysfunction16,46. This dysfunction allows more allergens and irritants to penetrate the skin, further triggering immune responses. Thus, reducing calcium intake may help mitigate excessive immune responses in certain populations, potentially lowering eczema prevalence through the mechanisms described above. Our study also indicates that increased calcium intake is significantly associated with higher eczema prevalence in groups such as non-Hispanic Whites and asthma patients. This phenomenon may reflect differences in immune responses, skin structure, or genetic background among these populations, making them more susceptible to the effects of dietary calcium intake changes47. Our study also shows that postmenopausal and elderly women exhibit a significantly higher prevalence of eczema with increased dietary calcium intake. This finding may be related to the physiological changes experienced during menopause and aging. As women age, estrogen levels decrease significantly. This reduction in estrogen weakens the skin’s moisture retention and barrier functions, making it more vulnerable to external allergens and irritants48. Research indicates that estrogen deficiency may also lead to an enhanced immune response to inflammation, exacerbating allergic reactions49. Against this background, increased calcium intake may further activate Th2 and Th17 cells, leading to the onset and exacerbation of eczema.
This study is the first to use representative large-sample data to investigate the association between calcium intake and eczema in an adult population. Our models were adjusted for a wide range of variables and utilized segmented regression to explore nonlinear relationships, providing new insights into the link between calcium and eczema. However, there are some limitations to our study. First, the cross-sectional design of this study does not allow for causal inferences; future prospective studies are needed to explore causal relationships. Second, the information on eczema was obtained through participant recall, which introduces potential recall bias. Third, dietary intake data were based on the 24-hour dietary recall method, and although two assessments were conducted, they still represent single time-point data, which may not fully capture long-term dietary intake patterns. Finally, the NHANES 2005–2006 data used in this study did not provide comprehensive information on calcium intake from supplements, and the collection method for supplements was not fully quantified. This limits our ability to assess the contribution of supplements to total calcium intake, which may influence the interpretation of the association between calcium intake and eczema.
The inverted U-shaped relationship found in this study provides new insights into the link between dietary calcium intake and eczema risk, suggesting that reducing calcium intake in specific populations may help lower the risk of allergic flare-ups. These findings highlight the need to consider the sensitivity of different populations to calcium intake when developing individualized dietary recommendations for the effective prevention and management of allergic diseases such as eczema.
This study is a secondary exploration of the NHANES public database. The data used in the manuscript can be accessed and downloaded from the website https://wwwn.cdc.gov/nchs/nhanes/search/default.aspx.
Strachan, D. P. et al. Worldwide time trends in prevalence of symptoms of rhinoconjunctivitis in children: global Asthma Network Phase I. Pediatr. Allergy Immunol. 33 (1), e13656. https://doi.org/10.1111/pai.13656 (2022).
Article PubMed MATH Google Scholar
Asher, M. I. et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases one and three repeat multicountry cross-sectional surveys. Lancet 368 (9537), 733–743. https://doi.org/10.1016/s0140-6736(06)69283-0 (2006).
Article PubMed MATH Google Scholar
Chalmers, J. R. et al. Daily emollient during infancy for prevention of eczema: the BEEP randomised controlled trial. Lancet 395 (10228), 962–972. https://doi.org/10.1016/s0140-6736(19)32984-8 (2020).
Article PubMed PubMed Central MATH Google Scholar
Kantor, R. & Silverberg, J. I. Environmental risk factors and their role in the management of atopic dermatitis. Expert Rev. Clin. Immunol. 13 (1), 15–26. https://doi.org/10.1080/1744666x.2016.1212660 (2017).
Article CAS PubMed MATH Google Scholar
Tétart, F. & Joly, P. Eczema in elderly people. Eur. J. Dermatol. 30 (6), 663–667. https://doi.org/10.1684/ejd.2020.3915 (2020).
Article PubMed MATH Google Scholar
Masuda, H. et al. Maternal dietary folate intake with folic acid supplements and wheeze and eczema in children aged 2 years in the Japan Environment and Children’s study. PLoS One. 17 (8), e0272968. https://doi.org/10.1371/journal.pone.0272968 (2022).
Article CAS PubMed PubMed Central Google Scholar
Leung, T. F. et al. Assessment of dietary food and nutrient intake and bone density in children with eczema. Hong Kong Med. J. 23 (5), 470–479. https://doi.org/10.12809/hkmj164684 (2017).
Article CAS PubMed MATH Google Scholar
Lim, J. J., Reginald, K., Say, Y. H., Liu, M. H. & Chew, F. T. Dietary protein intake and Associated risks for atopic dermatitis, intrinsic Eczema, and allergic sensitization among young Chinese adults in Singapore/Malaysia: key findings from a cross-sectional study. JID Innov. 3 (6), 100224. https://doi.org/10.1016/j.xjidi.2023.100224 (2023).
Article PubMed PubMed Central Google Scholar
Xu, J. & Li, H. Association between dietary antioxidants intake and childhood eczema: results from the NHANES database. J. Health Popul. Nutr. 43 (1), 12. https://doi.org/10.1186/s41043-024-00501-x (2024).
Article PubMed PubMed Central MATH Google Scholar
Miyake, Y., Sasaki, S., Tanaka, K. & Hirota, Y. Dairy food, calcium and vitamin D intake in pregnancy, and wheeze and eczema in infants. Eur. Respir J. 35 (6), 1228–1234. https://doi.org/10.1183/09031936.00100609 (2010).
Article CAS PubMed Google Scholar
Beggs, M. R., Bhullar, H., Dimke, H. & Alexander, R. T. The contribution of regulated colonic calcium absorption to the maintenance of calcium homeostasis. J. Steroid Biochem. Mol. Biol. 220, 106098. https://doi.org/10.1016/j.jsbmb.2022.106098 (2022).
Article CAS PubMed Google Scholar
Oda, Y., Tu, C. L., Menendez, A., Nguyen, T. & Bikle, D. D. Vitamin D and calcium regulation of epidermal wound healing. J. Steroid Biochem. Mol. Biol. 164, 379–385. https://doi.org/10.1016/j.jsbmb.2015.08.011 (2016).
Article CAS PubMed Google Scholar
Devlin, J., Stanton, R. H. & David, T. J. Calcium intake and cows’ milk free diets. Arch. Dis. Child. 64 (8), 1183–1184. https://doi.org/10.1136/adc.64.8.1183 (1989).
Article CAS PubMed PubMed Central MATH Google Scholar
Hildebrand, H., Simons, E., Kozyrskyj, A. L., Becker, A. B. & Protudjer, J. L. Calcium intake in children with Eczema and/or food allergy: a prospective cohort study. Nutrients 11 (12). https://doi.org/10.3390/nu11123039 (2019).
Elsori, D. H. & Hammoud, M. S. Vitamin D deficiency in mothers, neonates and children. J. Steroid Biochem. Mol. Biol. 175, 195–199. https://doi.org/10.1016/j.jsbmb.2017.01.023 (2018).
Article CAS PubMed Google Scholar
Lo, Y., Lin, L. Y. & Tsai, T. F. Use of calcium channel blockers in dermatology: a narrative review. Expert Rev. Clin. Pharmacol. 14 (4), 481–489. https://doi.org/10.1080/17512433.2021.1894128 (2021).
Article CAS PubMed MATH Google Scholar
Wei, J., Jaleel, T., MacLeod, A. S. & Ji, J. S. Inverted U-shaped relationship between vitamin D and ever-reported eczema in US adults. Allergy 74 (5), 964–975. https://doi.org/10.1111/all.13708 (2019).
Article CAS PubMed Google Scholar
Miyake, Y., Tanaka, K., Okubo, H., Sasaki, S. & Arakawa, M. Dairy food, calcium and vitamin D intake and prevalence of allergic disorders in pregnant Japanese women. Int. J. Tuberc Lung Dis. 16 (2), 255–261. https://doi.org/10.5588/ijtld.11.0173 (2012).
Article CAS PubMed Google Scholar
Jabbar-Lopez, Z. K. et al. The effect of water hardness on atopic eczema, skin barrier function: a systematic review, meta-analysis. Clin. Exp. Allergy. 51 (3), 430–451. https://doi.org/10.1111/cea.13797 (2021).
Article PubMed MATH Google Scholar
Curtin, L. R. et al. The National Health and Nutrition Examination Survey: Sample Design, 1999–2006. Vital Health Stat. 2 (155), 1–39 (2012).
MATH Google Scholar
Dwyer, J., Ellwood, K., Leader, N. P., Moshfegh, A. J. & Johnson, C. L. Integration of the Continuing Survey of Food Intakes by individuals and the National Health and Nutrition Examination Survey. J. Am. Diet. Assoc. 101 (10), 1142–1143. https://doi.org/10.1016/s0002-8223(01)00279-6 (2001).
Article CAS PubMed Google Scholar
Ahluwalia, N., Dwyer, J., Terry, A., Moshfegh, A. & Johnson, C. Update on NHANES Dietary Data: Focus on Collection, Release, Analytical considerations, and uses to inform Public Policy. Adv. Nutr. 7 (1), 121–134. https://doi.org/10.3945/an.115.009258 (2016).
Article PubMed PubMed Central Google Scholar
Wei, J. & Ji, J. S. Association of Serum Vitamins with Eczema in US adults (NHANES 2005–2006). Dermatology 236 (2), 179–182. https://doi.org/10.1159/000502642 (2020).
Article PubMed MATH Google Scholar
Haskell, W. L. et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med. Sci. Sports Exerc. 39 (8), 1423–1434. https://doi.org/10.1249/mss.0b013e3180616b27 (2007).
Article PubMed MATH Google Scholar
Wu, Z. et al. A cross-sectional study of smoking and depression among US adults: NHANES (2005–2018). Front. Public. Health. 11, 1081706. https://doi.org/10.3389/fpubh.2023.1081706 (2023).
Article PubMed PubMed Central Google Scholar
Tian, Y. et al. Alcohol consumption and all-cause and cause-specific mortality among US adults: prospective cohort study. BMC Med. 21 (1), 208. https://doi.org/10.1186/s12916-023-02907-6 (2023).
Article PubMed PubMed Central MATH Google Scholar
Swed, S. et al. Asthma prevalence among United States population insights from NHANES data analysis. Sci. Rep. 14 (1), 8059. https://doi.org/10.1038/s41598-024-58429-5 (2024).
Article ADS CAS PubMed PubMed Central MATH Google Scholar
Johnson, C. L., Dohrmann, S. M., Burt, V. L. & Mohadjer, L. K. National health and nutrition examination survey: sample design, 2011–2014. Vital Health Stat. 2 (162):1–33. (2014).
Zipf, G. et al. National health and nutrition examination survey: plan and operations, 1999–2010. Vital Health Stat. 1 (56), 1–37 (2013).
MATH Google Scholar
de Luis, D. A. et al. Dietary intake in patients with asthma: a case control study. Nutrition 21 (3), 320–324. https://doi.org/10.1016/j.nut.2004.06.027 (2005).
Article PubMed MATH Google Scholar
Gomes, F. et al. Calcium supplementation for the prevention of hypertensive disorders of pregnancy: current evidence and programmatic considerations. Ann. N Y Acad. Sci. 1510 (1), 52–67. https://doi.org/10.1111/nyas.14733 (2022).
Article ADS CAS PubMed PubMed Central MATH Google Scholar
Hyppönen, E. et al. Infant vitamin d supplementation and allergic conditions in adulthood: northern Finland birth cohort 1966. Ann. N Y Acad. Sci. 1037, 84–95. https://doi.org/10.1196/annals.1337.013 (2004).
Article ADS CAS PubMed Google Scholar
Gale, C. R. et al. Maternal vitamin D status during pregnancy and child outcomes. Eur. J. Clin. Nutr. 62 (1), 68–77. https://doi.org/10.1038/sj.ejcn.1602680 (2008).
Article CAS PubMed MATH Google Scholar
Bi, W. G. et al. Association between Vitamin D Supplementation during Pregnancy and offspring growth, morbidity, and mortality: a systematic review and Meta-analysis. JAMA Pediatr. 172 (7), 635–645. https://doi.org/10.1001/jamapediatrics.2018.0302 (2018).
Article PubMed PubMed Central MATH Google Scholar
Liu, T. et al. The IL-23/IL-17 pathway in inflammatory skin diseases: from bench to Bedside. Front. Immunol. 11, 594735. https://doi.org/10.3389/fimmu.2020.594735 (2020).
Article CAS PubMed PubMed Central Google Scholar
Shan, M. et al. Secreted IgD amplifies humoral T helper 2 cell responses by binding basophils via Galectin-9 and CD44. Immunity 49 (4), 709–24e8. https://doi.org/10.1016/j.immuni.2018.08.013 (2018).
Article MathSciNet CAS PubMed PubMed Central Google Scholar
Park, H. J., Lee, S. W. & Hong, S. Regulation of allergic Immune responses by Microbial metabolites. Immune Netw. 18 (1), e15. https://doi.org/10.4110/in.2018.18.e15 (2018).
Article PubMed PubMed Central MATH Google Scholar
Barthels, C. et al. CD40-signalling abrogates induction of RORγt(+) Treg cells by intestinal CD103(+) DCs and causes fatal colitis. Nat. Commun. 8, 14715. https://doi.org/10.1038/ncomms14715 (2017).
Article ADS PubMed PubMed Central MATH Google Scholar
Johansson, S., Lönnqvist, A., Ostman, S., Sandberg, A. S. & Wold, A. E. Long-chain polyunsaturated fatty acids are consumed during allergic inflammation and affect T helper type 1 (Th1)- and Th2-mediated hypersensitivity differently. Clin. Exp. Immunol. 160 (3), 411–419. https://doi.org/10.1111/j.1365-2249.2010.04107.x (2010).
Article CAS PubMed PubMed Central Google Scholar
Dong, X. & Dong, X. Peripheral and Central Mechanisms of Itch. Neuron 98 (3), 482–494. https://doi.org/10.1016/j.neuron.2018.03.023 (2018).
Article CAS PubMed PubMed Central MATH Google Scholar
Ross, S. H. & Cantrell, D. A. Signaling and function of Interleukin-2 in T lymphocytes. Annu. Rev. Immunol. 36, 411–433. https://doi.org/10.1146/annurev-immunol-042617-053352 (2018).
Article CAS PubMed PubMed Central MATH Google Scholar
Evans, R. D. R. et al. Inherited salt-losing tubulopathies are associated with immunodeficiency due to impaired IL-17 responses. Nat. Commun. 11 (1), 4368. https://doi.org/10.1038/s41467-020-18184-3 (2020).
Article ADS CAS PubMed PubMed Central MATH Google Scholar
Matheu, V., Bäck, O., Mondoc, E. & Issazadeh-Navikas, S. Dual effects of vitamin D-induced alteration of TH1/TH2 cytokine expression: enhancing IgE production and decreasing airway eosinophilia in murine allergic airway disease. J. Allergy Clin. Immunol. 112 (3), 585–592. https://doi.org/10.1016/s0091-6749(03)01855-4 (2003).
Article CAS PubMed Google Scholar
Pryshchep, S., Goronzy, J. J., Parashar, S. & Weyand, C. M. Insufficient deactivation of the protein tyrosine kinase lck amplifies T-cell responsiveness in acute coronary syndrome. Circ. Res. 106 (4), 769–778. https://doi.org/10.1161/circresaha.109.206052 (2010).
Article CAS PubMed Google Scholar
Na, N. et al. Carbamylated erythropoietin regulates immune responses and promotes long-term kidney allograft survival through activation of PI3K/AKT signaling. Signal. Transduct. Target. Ther. 5 (1), 194. https://doi.org/10.1038/s41392-020-00232-5 (2020).
Article CAS PubMed PubMed Central Google Scholar
Miao, Q. et al. SOX11 and SOX4 drive the reactivation of an embryonic gene program during murine wound repair. Nat. Commun. 10 (1), 4042. https://doi.org/10.1038/s41467-019-11880-9 (2019).
Article ADS CAS PubMed PubMed Central MATH Google Scholar
Sy, H. & Ditto, A. M. Racial and Ethnic Disparity in Allergic Diseases in the United States: Example of a large country with a Diverse Population. In: (ed Mahdavinia, M.) Health Disparities in Allergic Diseases: An Evidence-Based Look at Causes, Conditions, and Outcomes. Cham: Springer International Publishing; 73–96. (2020).
Chapter Google Scholar
Tang, Y. et al. Systemic immune-inflammation index and bone mineral density in postmenopausal women: a cross-sectional study of the national health and nutrition examination survey (NHANES) 2007–2018. Front. Immunol. 13, 975400. https://doi.org/10.3389/fimmu.2022.975400 (2022).
Article CAS PubMed PubMed Central Google Scholar
Myung, S. K., Kim, H. B., Lee, Y. J., Choi, Y. J. & Oh, S. W. Calcium supplements and risk of Cardiovascular Disease: a Meta-analysis of clinical trials. Nutrients 13 (2). https://doi.org/10.3390/nu13020368 (2021).
Download references
Thanks to all NHANES researchers, staff, and participants for their contributions to the completion of this analysis.
This research was supported by the Wuxi Science and Technology Plan Project (Y20212016); Research project of Wuxi Health Commission (M202353); Jiangsu University Senior Talent Fund Grant Program (23JDG033).
†Qianjie Wu and Zitao Guo contribute equally to this study.
Wuxi Medical College, Jiangnan University, Wuxi, China
Qianjie Wu
School of Food and Biological Engineering, Jiangsu University, Zhenjiang, 212013, China
Zitao Guo
Department of Dermatology, Affiliated Hospital of Jiangnan University, 1000 Hefeng Road, Binhu District, WuxiCity, 214122, China
Na Zhang & Dake Dong
You can also search for this author in PubMed Google Scholar
You can also search for this author in PubMed Google Scholar
You can also search for this author in PubMed Google Scholar
You can also search for this author in PubMed Google Scholar
QW and ZG were responsible for writing and revising the paper. DD was responsible for conceptualizing the article and participating in the design.QW and ZG were responsible for statistically analyzing the data. QW and ZG replicated the collection of medical records and data organization. NZ contributed to the conception design and review of the manuscript or critical revision for important intellectual content.All authors approved the final version, and agree to be accountable for all aspects of the work.
Correspondence to Dake Dong.
The authors declare no competing interests.
This study was a secondary analysis of publicly available data, written informed consent was obtained from participants for the recording of all data, and ethical approvals for the study data were obtained from the Ethics Review Board of the National Center for Health Statistics (NCHS); for all information on these ethical approvals, please visit the following website: https://www.cdc.gov/nchs/nhanes/irba98.htm.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Below is the link to the electronic supplementary material.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
Reprints and permissions
Wu, Q., Guo, Z., Zhang, N. et al. Correlation between dietary calcium intake and eczema in American adult population. Sci Rep 14, 31270 (2024). https://doi.org/10.1038/s41598-024-82723-x
Download citation
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-82723-x
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Advertisement
© 2024 Springer Nature Limited
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
