Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
Advertisement
Scientific Reports volume 15, Article number: 222 (2025)
Metrics details
To investigate the relationship between the Chinese Visceral Adiposity Index (CVAI) and the risk of developing Benign Prostatic Hyperplasia (BPH). Using data from the China Health and Retirement Longitudinal Study (CHARLS), we included 3,295 men aged 45 years and older. Multivariate logistic regression and restricted cubic spline models were employed to analyze the association between CVAI and the risk of BPH. During the 4-year follow-up period, 267 cases of BPH were identified. CVAI was positively associated with the risk of developing BPH (OR = 1.23, 95% CI: 1.07–1.42), with a significant dose-response relationship (P < 0.001). Stratified analysis showed that the effect of CVAI on BPH risk was consistent across various subgroups. There is a positive correlation between CVAI and the risk of developing BPH. Managing visceral fat content and maintaining a healthy fat distribution pattern may help reduce the risk of BPH.
Benign Prostatic Hyperplasia (BPH) refers to the non-cancerous enlargement of the prostate gland and is one of the most common conditions among middle-aged and elderly men. With the acceleration of the aging process, the incidence of BPH is also increasing. In China, the prevalence of BPH exceeds 50% among men aged 60 and over, and reaches as high as 83% among men aged 80 and over1. According to statistics, there were 11.26 million new cases of BPH worldwide in 2019, leading to 1.86 million Disability-Adjusted Life Years (DALYs)2. As a significant public health concern, BPH severely impacts the quality of life and health status of middle-aged and elderly patients, while also increasing global healthcare expenditures3,4. BPH can lead to various adverse effects, such as Lower Urinary Tract Symptoms (LUTS), which include frequent urination, urgency, nocturia, weak urine stream, dribbling, and incomplete bladder emptying5. Studies have reported that moderate to severe LUTS can increase the risk of adverse cardiac events6. BPH can cause urethral obstruction, leading to urinary retention and urinary tract infections. Prolonged urinary obstruction can result in decreased bladder contractility, exacerbating difficulties in urination. Persistent urinary obstruction and bladder dysfunction can further lead to hydronephrosis and renal insufficiency, and in severe cases, renal failure7,8. Therefore, identifying high-risk populations for BPH and implementing early interventions and treatments are crucial for reducing its incidence and improving overall health outcomes.
The Chinese Visceral Adiposity Index (CVAI) is a novel adiposity assessment model tailored for the Chinese population9. It is based on age, Body Mass Index (BMI), Waist Circumference (WC), Triglycerides (TG), and High-Density Lipoprotein (HDL) levels, and it can be used to estimate visceral fat content and predict the risk of metabolic diseases9. Currently, the relationship between CVAI and BPH remains unclear. Therefore, we conducted a prospective study using data from the China Health and Retirement Longitudinal Study (CHARLS)10 database to investigate the association between CVAI and the risk of developing BPH.
This study utilized the baseline data from 2011 and follow-up data from 2015 from the CHARLS database (http://charls.pku.edu.cn/), which is publicly accessible. CHARLS is a large, nationwide, interdisciplinary survey covering 450 villages/communities in 28 provinces (autonomous regions and municipalities) in China, targeting participants aged 45 years and older. The survey encompasses a wide range of topics, including demographic information, family structure, health status, healthcare and insurance, employment and pensions, income and expenditures, housing conditions, and laboratory test results. Participants provided fasting venous blood samples after fasting for over 12 h, which were immediately analyzed for complete blood cell counts on-site. Whole blood samples were stored at 4 °C, and remaining samples were transported to a central laboratory for further analysis of glucose, Total Cholesterol (TC), TG, Low-Density Lipoprotein Cholesterol (LDL-C), and High-Density Lipoprotein Cholesterol (HDL-C) levels using enzymatic colorimetric methods. Diagnostic information on BPH was collected from the 2015 follow-up data.
The CHARLS study received approval from the Institutional Review Board (IRB) of Peking University in 2008 (Approval No. IRB00001052-11015). This study strictly adhered to all CHARLS protocols and requirements. All participants voluntarily signed informed consent forms prior to the survey.
For this study, the inclusion criteria were defined as follows: age ≥ 45 years, complete socio-demographic information (including educational level, marital status, and residence), and availability of both 2011 baseline and 2015 follow-up data. Participants with missing values for exposure or outcome variables, as well as those who were diagnosed with BPH at baseline, were excluded from the analysis.
The CVAI equation is derived from a regression model that was validated in previous studies9. The equation incorporates five variables: age, BMI, WC, TG, and HDL-C. These variables were chosen because they are closely linked to visceral fat accumulation and metabolic disturbances, which are key components of metabolic syndrome and contribute to BPH pathogenesis. The equation is designed to capture the combined effects of these factors, providing a composite measure of visceral fat and metabolic risk. While CVAI is an indirect estimate of visceral fat, it has been shown to correlate well with direct imaging methods, such as CT or MRI, and has been validated in large-scale cohort studies.
The CVAI score for Chinese males is calculated using the following formula: CVAI = − 267.93 + 0.68×Age + 0.03×BMI + 4.00×WC + 22.00×log10(TG) − 16.32×HDL-C.
Men who were not diagnosed with BPH during the 2011 baseline survey but were newly diagnosed with BPH in the 2015 follow-up were defined as BPH cases. BPH diagnosis was based on self-reported data from participants. Specifically, participants were asked in a questionnaire whether they had ever been diagnosed with a prostate illness, such as prostate hyperplasia, by a healthcare professional. The diagnosis was confirmed through participant responses to questions regarding prior medical evaluations and physician diagnoses10.
The analysis included socio-demographic characteristics, lifestyle factors, and physical examination indicators as covariates. Socio-demographic variables comprised age, educational level (middle school or below / high school / college or above), residence (urban / rural), and marital status (married / unmarried or divorced or widowed). Lifestyle factors included smoking status (never smoked / currently smoking), alcohol consumption frequency (never / less than once a month / at least once a month), and sleep duration. This information was collected through self-reported questionnaires completed by participants under the guidance of trained interviewers. Physical examination indicators included Systolic Blood Pressure (SBP) and Diastolic Blood Pressure (DBP), measured three times using an Omron HEM-7200 electronic sphygmomanometer, with the average values used for analysis.
Continuous variables following a normal distribution were expressed as mean ± standard deviation (SD), while those with skewed distribution were expressed as median (interquartile range, IQR). Categorical variables were presented as frequencies (percentages). Differences in baseline characteristics and BPH incidence across CVAI quartiles (Q1-Q4) were compared using one-way ANOVA, Kruskal-Wallis H test, or chi-square test as appropriate. Three logistic regression models were employed to evaluate the association between CVAI and BPH risk. CVAI was analyzed both as a continuous variable (per SD increase) and as a categorical variable (quartiles). Model 1 was unadjusted; Model 2 adjusted for socio-demographic factors such as age, education level, residence, and marital status; Model 3 further adjusted for lifestyle and physiological indicators, including smoking status, alcohol consumption frequency, sleep duration, SBP, and diastolic DBP. For the stratified analysis, we employed Model 3 (adjusting for confounders) and assessed the outcome variable as categorized. The strength of the association was expressed as odds ratios (ORs) with 95% confidence intervals (CIs). Interaction analyses were conducted by including interaction terms [CVAI × (covariate)] in the models to examine the potential effect modification of socio-demographic characteristics and lifestyle factors on the relationship between CVAI and BPH. Additionally, restricted cubic spline (RCS) regression was used to explore the non-linear dose-response relationship between CVAI and BPH risk.
All analyses were performed using R Statistical Software (Version 4.2.2, http://www.R-project.org, The R Foundation) and Free Statistics analysis platform (Version 2.0, Beijing, China). The “rms” package was utilized for restricted cubic spline modeling; the “rcs” function was used to define the restricted cubic spline terms for the independent variable and to fit the regression model. All statistical tests were two-sided, and a p-value < 0.05 was considered statistically significant.
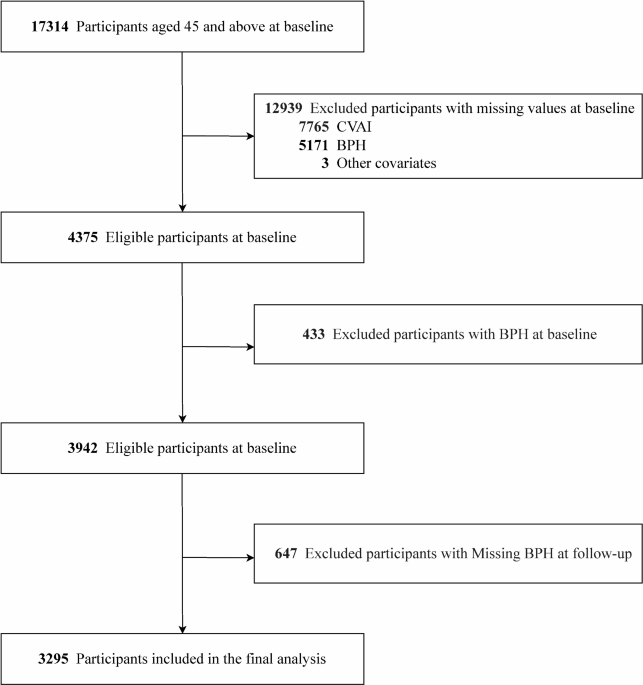
A total of 3,295 eligible participants were included in the final analysis after excluding participants with missing data or those diagnosed with BPH at baseline (Fig. 1), with an average age of 59.4 ± 8.7 years. During the follow-up period, 297 men developed BPH. The baseline median CVAI for those who developed BPH was 99.5 (70.6, 133.9), compared to 85.1 (57.1, 119.8) for those who did not develop BPH. Baseline characteristics of BPH patients differed significantly from those without BPH, with BPH patients being older, having lower educational levels, more likely to reside in rural areas, and having higher BMI and waist circumference (Table 1).
Flowchart of participants selection.
Three logistic regression models were constructed to evaluate the association between CVAI and BPH risk, with adjustments for different covariates. After adjusting for covariates, the analysis was conducted using Models 1, 2, and 3. In Model 3, CVAI was significantly associated with BPH risk, with an adjusted OR of 1.93 (95% CI: 1.32–2.84). When CVAI was included in the model as a continuous variable (per SD increase), it was positively associated with BPH risk (OR = 1.23, 95% CI: 1.07–1.42, p = 0.004). Furthermore, the risk of BPH increased across CVAI quartiles (p < 0.001) (Table 2; Fig. 2).
Restricted cubic spline of the association between CVAI and the risk of BPH. The model was adjusted for age, education level, location and marital status, smoking status, drinking status, sleep time, SBP, and DBP. The plot shows a relationship between CVAI and the risk of BPH. The reference point is the median of all the data.
To explore the heterogeneity of the association between CVAI and BPH risk across different subgroups, we conducted a stratified analysis based on age, education level, and residence. The results of the stratified analysis indicated that the positive association between CVAI and BPH risk was consistent across all subgroups, with no significant effect modification observed. Within each subgroup, higher CVAI values were associated with an increased risk of BPH. For the stratified analysis, we employed Model 3 (adjusting for confounders) and assessed the outcome variable as categorized (Fig. 3).
Forest plot of stratified analysis of the association of CVAI with the risk of BPH.
This study, based on data from the CHARLS, investigated the relationship between the CVAI and the risk of developing BPH. The findings revealed a significant positive correlation between CVAI and BPH incidence, demonstrating a clear dose-response relationship. As CVAI quartiles increased, the risk of BPH progressively rose, suggesting that CVAI might be an independent risk factor for BPH. Further stratified analysis showed that the impact of CVAI on BPH risk was consistent across subgroups defined by age, education level, and residence, with no significant effect modification detected. This study contributes to the current understanding of BPH by identifying CVAI as a potentially valuable marker for BPH risk stratification. This study is the first to establish a positive association between CVAI and the risk of BPH, indicating that managing visceral fat content and maintaining a healthy fat distribution pattern may help reduce the incidence of BPH.
CVAI is not only a marker of visceral fat but also incorporates factors such as insulin resistance and inflammatory markers11, both of which are implicated in the development of BPH12. Recent studies have emphasized the critical role of insulin resistance and inflammation in BPH development11. These metabolic factors promote prostate cell proliferation and inhibit cell apoptosis13, thus facilitating the growth of prostatic tissue14. Our results align with these findings, suggesting that CVAI can offer more nuanced insights into the relationship between obesity, metabolic dysfunction, and BPH risk. In addition to metabolic dysfunction and inflammation, hormonal changes also contribute significantly to BPH development15. The conversion of circulating testosterone to estrogen in prostate tissue disrupts the balance of these hormones, which further promotes prostatic hyperplasia16. Furthermore, studies have shown that aging and changes in steroid hormone levels are major factors influencing prostate enlargement17. Interestingly, reducing body fat percentage in obese individuals has been shown to alleviate male LUTS, highlighting the importance of managing obesity and metabolic dysfunction in preventing or mitigating BPH. CVAI, as a more comprehensive marker of visceral fat and metabolic dysfunction, offers important insights into the pathophysiology of BPH. Our study supports the potential of CVAI as an effective tool for identifying individuals at higher risk for BPH, particularly those with metabolic disturbances that predispose them to prostate enlargement. Dietary factors, such as a preference for fatty foods, may play a role in BPH development, and while our study did not account for these variables, future research should consider their potential impact on BPH risk18. Additionally, family history is an established risk factor for BPH, and while we did not have access to detailed family history data, this factor could confound the observed associations. We recommend that future studies incorporate dietary habits and family history to better isolate the effects of metabolic indices like CVAI on BPH. Future survey should explore the role of CVAI in larger, more diverse populations and investigate its utility in clinical risk stratification for BPH.
While our study provides valuable insights into the association between CVAI and BPH, several limitations should be acknowledged. First, due to the observational design of the study, we cannot establish causality between CVAI and the risk of BPH. Second, the reliance on self-reported BPH diagnoses introduces the potential for misclassification and recall bias, which could affect the accuracy of the findings. Third, the cross-sectional nature of our study restricts our ability to assess changes in CVAI or BPH risk over time, limiting our understanding of the long-term effects of these factors. Additionally, while we adjusted for a range of confounders, there may still be residual confounding factors that were not captured in the analysis. This study did not account for potential confounders such as dietary preferences (e.g., fatty food consumption) or family history of BPH, both of which may influence the development of the disease. Future studies should consider these factors to provide a more comprehensive understanding of the relationship between CVAI and BPH.
The generalizability of our findings is also limited by the study’s sample population, which consists solely of Chinese men aged 45 and older. Further research is needed to determine whether these results are applicable to younger populations or other demographic groups. These limitations highlight the need for further studies, ideally with longitudinal data and more diverse cohorts, to confirm the validity and broader applicability of our findings. Although the current study offers valuable insights, future research should aim to validate these results using independent datasets. Conducting the same analysis on external validation cohorts would increase the robustness and generalizability of our conclusions, allowing them to be more confidently applied to a broader range of populations and clinical settings.
This study is the first to establish a positive correlation between the CVAI and the risk of developing BPH, demonstrating a clear dose-response relationship. These findings suggest that managing visceral fat content and maintaining a healthy fat distribution pattern may help reduce the risk of BPH, offering new insights for the early prevention and intervention of BPH.
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Chinese Visceral Adiposity Index
Benign Prostatic Hyperplasia
China Health and Retirement Longitudinal Study
Disability-Adjusted Life Years
Lower Urinary Tract Symptoms
Body Mass Index
Waist Circumference
Triglycerides
High-Density Lipoprotein
Total Cholesterol
Low-Density Lipoprotein Cholesterol
High-Density Lipoprotein Cholesterol
Institutional Review Board
Systolic Blood Pressure
Diastolic Blood Pressure
Odds Ratio
Confidence Intervals
Gu, F. L., Xia, T. L. & Kong, X. T. Preliminary study of the frequency of benign prostatic hyperplasia and prostatic cancer in China. Urology 44, 688–691. https://doi.org/10.1016/s0090-4295(94)80207-6 (1994).
Article CAS PubMed Google Scholar
Xu, X. F. et al. Global, Regional, and National Incidence and Year lived with disability for Benign Prostatic Hyperplasia from 1990 to 2019. Am. J. Mens Health. 15, 15579883211036786. https://doi.org/10.1177/15579883211036786 (2021).
Article PubMed PubMed Central Google Scholar
Devlin, C. M., Simms, M. S. & Maitland, N. J. Benign prostatic hyperplasia – What do we know? BJU Int. 127, 389–399. https://doi.org/10.1111/bju.15229 (2021).
Article CAS PubMed Google Scholar
Taub, D. A. & Wei, J. T. The economics of benign prostatic hyperplasia and lower urinary tract symptoms in the United States. Curr. Urol. Rep. 7, 272–281. https://doi.org/10.1007/s11934-996-0006-0 (2006).
Article PubMed Google Scholar
Dornbier, R., Pahouja, G., Branch, J. & McVary, K. T. The New American Urological Association Benign Prostatic Hyperplasia Clinical Guidelines: 2019 update. Curr. Urol. Rep. 21, 32. https://doi.org/10.1007/s11934-020-00985-0 (2020).
Article PubMed Google Scholar
Gacci, M. et al. Male lower urinary tract symptoms and cardiovascular events: A systematic review and Meta-analysis. Eur. Urol. 70, 788–796. https://doi.org/10.1016/j.eururo.2016.07.007 (2016).
Article PubMed Google Scholar
Ottaiano, N., Shelton, T., Sanekommu, G. & Benson, C. R. Surgical complications in the management of Benign Prostatic Hyperplasia Treatment. Curr. Urol. Rep. 23, 83–92. https://doi.org/10.1007/s11934-022-01091-z (2022).
Article PubMed Google Scholar
Lim, K. B. Epidemiology of clinical benign prostatic hyperplasia. Asian J. Urol. 4, 148–151. https://doi.org/10.1016/j.ajur.2017.06.004 (2017).
Article PubMed PubMed Central Google Scholar
Xia, M. F. et al. A indicator of visceral adipose dysfunction to evaluate metabolic health in adult Chinese. Sci. Rep. 6, 38214. https://doi.org/10.1038/srep38214 (2016).
Article ADS CAS PubMed PubMed Central Google Scholar
Zhao, Y., Hu, Y., Smith, J. P., Strauss, J. & Yang, G. Cohort profile: The China health and retirement longitudinal study (CHARLS). Int. J. Epidemiol. 43, 61–68 (2014).
Article PubMed Google Scholar
Cui, Y., Wang, H. & Wang, Y. Plasma metabolites as mediators in the relationship between inflammation-related proteins and benign prostatic hyperplasia: Insights from mendelian randomization. Sci. Rep. 14, 26152 (2024).
Article CAS PubMed PubMed Central Google Scholar
De Nunzio, C., Presicce, F. & Tubaro, A. Inflammatory mediators in the development and progression of benign prostatic hyperplasia. Nat. Reviews Urol. 13, 613–626 (2016).
Article Google Scholar
Parsons, J. K., Sarma, A. V., McVary, K. & Wei, J. T. Obesity and benign prostatic hyperplasia: Clinical connections, emerging etiological paradigms and future directions. J. Urol. 189, 102–S106 (2013).
Article Google Scholar
Gandaglia, G. et al. The role of chronic prostatic inflammation in the pathogenesis and progression of benign prostatic hyperplasia (BPH). BJU Int. 112 (2013).
Yin, Z., Yang, J. R., Rao, J. M., Song, W. & Zhou, K. Q. Association between benign prostatic hyperplasia, body mass index, and metabolic syndrome in Chinese men. Asian J. Androl. 17, 826–830 (2015).
Article PubMed PubMed Central Google Scholar
Vignozzi, L. et al. Benign prostatic hyperplasia: A new metabolic disease? J. Endocrinol. Investig. 37, 313–322 (2014).
Article CAS Google Scholar
La Vignera, S., Condorelli, R., Russo, G., Morgia, G. & Calogero, A. Endocrine control of benign prostatic hyperplasia. Andrology 4, 404–411 (2016).
Article PubMed Google Scholar
Li, Y. et al. Effects of inflammatory responses, apoptosis, and STAT3/NF-κB-and Nrf2-mediated oxidative stress on benign prostatic hyperplasia induced by a high-fat diet. Aging (Albany NY). 11, 5570 (2019).
Article CAS PubMed Google Scholar
Download references
We sincerely thank all members of our research team for their invaluable support and contributions throughout this study. We are grateful to the CHARLS project and Peking University for providing the dataset that made this research possible.
None.
Bing Li and Junping Li have contributed equally to this work.
Department of Urology, Tianjin Medical University General Hospital, Tianjin, China
Bing Li
Department of Oncology, Zibo City Municipal Hospital, Zibo, China
Junping Li
Department of Orthopaedics, Tianjin Medical University General Hospital, Tianjin, China
Chao Sun
Department of Cardiovascular surgery, Tianjin Medical University General Hospital, Tianjin, China
Yaodong Sun
Graduate School of Tianjin Medical University, Tianjin Medical University, Tianjin, China
Zhiqiang Zhang
You can also search for this author in PubMed Google Scholar
You can also search for this author in PubMed Google Scholar
You can also search for this author in PubMed Google Scholar
You can also search for this author in PubMed Google Scholar
You can also search for this author in PubMed Google Scholar
BL and ZZ: Conceptualization and design of the study, data acquisition and interpretation, drafting the manuscript. CS and YS: Conceptualization and design of the study, formal analysis, and methodology. JL: Preparation of figures and tables, drafting the manuscript. All authors contributed to the final version of the manuscript and approved it.
Correspondence to Zhiqiang Zhang.
The authors declare no competing interests.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
Reprints and permissions
Li, B., Li, J., Sun, C. et al. Association between the Chinese Visceral Adiposity Index and the risk of Benign Prostatic Hyperplasia: a national prospective cohort study. Sci Rep 15, 222 (2025). https://doi.org/10.1038/s41598-024-83960-w
Download citation
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-83960-w
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Advertisement
© 2025 Springer Nature Limited
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.